
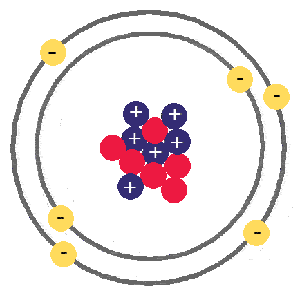
Protons and neutrons have approximately the same mass, about 1.67 × 10 -24 grams. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The most common isotope of hydrogen (H) is the only exception and is made of one proton and one electron with no neutrons.įigure 2.2 Elements, such as helium, depicted here, are made up of atoms. Atoms contain protons, electrons, and neutrons, among other subatomic particles. The atom's outermost region holds its electrons in orbit around the nucleus, as Figure 2.2 illustrates. We cannot break down gold atoms into anything smaller while still retaining the properties of gold.Īn atom is composed of two regions: the nucleus, which is in the atom's center and contains protons and neutrons.
A gold coin is simply a very large number of gold atoms molded into the shape of a coin and contains small amounts of other elements known as impurities. For example, one gold atom has all of the properties of gold, like its chemical reactivity. An atom is the smallest unit of matter that retains all of the element's chemical properties. To understand how elements come together, we must first discuss the element's smallest component or building block, the atom. In spite of their differences in abundance, all elements and the chemical reactions between them obey the same chemical and physical laws regardless of whether they are a part of the living or nonliving world. For example, the atmosphere is rich in nitrogen and oxygen but contains little carbon and hydrogen, while the earth’s crust, although it contains oxygen and a small amount of hydrogen, has little nitrogen and carbon. In the nonliving world, elements are found in different proportions, and some elements common to living organisms are relatively rare on the earth as a whole, as Table 2.1 shows. The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N). For example, the symbol for sodium is Na, referring to natrium, the Latin word for sodium. Other elements’ chemical symbols derive from their Latin names. Some elements follow the English term for the element, such as C for carbon and Ca for calcium. The remaining elements are unstable and require scientists to synthesize them in laboratories.Įach element is designated by its chemical symbol, which is a single capital letter or, when the first letter is already “taken” by another element, a combination of two letters. There are 118 elements, but only 98 occur naturally.

Elements are unique forms of matter with specific chemical and physical properties that cannot break down into smaller substances by ordinary chemical reactions. Matter is any substance that occupies space and has mass. Explain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organismsĪt its most fundamental level, life is made up of matter.



 0 kommentar(er)
0 kommentar(er)
